Journal of Civil Engineering and Environmental Sciences
Inhibitive Effect of Mangifera Indica Extract on Mild Steel in Hydrochloric Acid Solution
Olisakwe HC*, Osazuwa OK, Chukwuneke JL and Ezeanyanwu CS
Department of Mechanical Engineering, Nnamdi Azikiwe University, Awka, Nigeria
Cite this as
Olisakwe HC, Osazuwa OK, Chukwuneke JL, Ezeanyanwu CS. Inhibitive Effect of Mangifera Indica Extract on Mild Steel in Hydrochloric Acid Solution. J Civil Eng Environ Sci. 2024;10(2): 067-072. Available from: 10.17352/2455-488X.000087Copyright License
© 2024 Olisakwe HC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.This research investigated the corrosion inhibition potential of Mangifera Indica Peel Extract (MIPE) for mild steel in a 1 M HCl solution. The study explored the effects of extract concentration, solution temperature, and immersion time on the inhibition potential of MIPE using weight loss measurements at extract concentrations of 0, 1.0, 1.5, and 2.0 g/L, temperatures of 303 K and 323 K, and immersion times of 1, 2, 4, 6, and 8 h. Experimental results showed that MIPE significantly reduced the corrosion rate of mild steel, with maximum inhibition efficiency reaching 97.26% and 94.83% at 2.0 g/L MIPE concentration and solution temperatures of 303 K and 323 K, respectively. The uninhibited mild steel experienced increased corrosion rates with rising temperatures and longer immersion times. The inhibition efficiency of MIPE improved with higher extract concentrations and immersion periods. These findings underscore the potential of MIPE as an effective and environmentally friendly corrosion inhibitor in acidic environments.
Introduction
Corrosion, or metal deterioration, is a significant issue in a variety of industries, including transportation, manufacturing, and infrastructure [1-3]. Corrosion has a significant financial impact, costing hundreds of billions of dollars per year [4,5]. As a result, corrosion must be effectively controlled due to its negative consequences, which include increased maintenance costs, safety hazards, and structural degradation. There are numerous corrosion controls present. Nonetheless, there has recently been an increase in interest in researching sustainable and environmentally friendly methods of corrosion prevention, such as the use of plant extracts as corrosion inhibitors [5,6]. Mild steel is an iron-carbon alloy with trace amounts of silicon, sulfur, and phosphorus. According to Wei, et al. [7], mild steel is widely used in the petrochemical, construction, marine, chemical, and metallurgical industries for the production of automobile body components, ships, pipelines, structural shapes, buildings, bridges, railway lines, and tin cans, as well as some pots and pans used for cooking. However, it is corrosive in a variety of environments, particularly acidic ones. Tang, et al. [8] found that mild steel is widely used in a variety of industries for crude oil refining, acid pickling, industrial cleaning, acid descaling, oil recovery, and petrochemical processes. Ramezanzadeh, et al. [2] discovered that Hydrochloric Acid (HCl) is widely used in a variety of industries as a descaling and cleaning agent. Mild steel is used to make a variety of components and structures, including automobile body parts, ships, pipelines, structural shapes, buildings, bridges, railway lines, tin cans, and even cooking pots and pans [4,9].
Plant extracts contain a variety of bioactive components, including flavonoids, alkaloids, polyphenols, and organic acids, all of which have inherent antioxidant and anticorrosive properties [10-12]. An extract is a mixture of a plant’s active ingredients or parts combined with a solvent-acting medium. The polarity of the solvent used in the procedures or methods (Soxhlet and maceration), among other things, affects the extraction yields. The extract’s active principles provide the properties for a specific function. Thus, certain benefits can be linked to a particular plant based on its active principles and concentrations. The main benefits of these extracts are their antiviral, antibacterial, anti-inflammatory, and antioxidant properties [13-15]. Furthermore, plant extracts are thought to be a more sustainable and environmentally friendly alternative to traditional corrosion inhibitors. Because they are derived from natural and renewable sources, artificial chemicals are not required, and the number of hazardous materials released into the environment is reduced [16-18]. Reducing risks to human and environmental health. Plant extracts are also less expensive and more readily available. This is due to the abundance and accessibility of many plant parts, including peels, leaves, barks, and stems [1,13,19]. Mango (Mangifera indica L.) is widely recognized for its distinct flavor, aroma, and health benefits [1,20,21]. However, a significant amount of mango peels are discarded during mango processing for various food products. Although these peels are frequently discarded, they contain valuable bioactive compounds that can be used in other applications. Mango peels are known to contain antioxidant phytochemicals such as polyphenols, gallic acid, flavonoids, and organic acids [2,4,5]. The use of mango peels as a corrosion inhibitor has the added benefit of providing an environmentally friendly solution while also creating value from this agricultural waste material by utilizing the bioactive compounds found in the peels.
Acidic solutions are suitable for testing the efficacy of corrosion inhibitors because they are known to accelerate the corrosion process, particularly strong acids such as (hydrochloric acid) HCl [22-24]. Mango peel extract’s potential as a corrosion inhibitor in practical applications can be better understood by studying its inhibitory effects on mild steel corrosion in an acidic environment [25-28]. To determine the optimal conditions for inhibition, test its performance at various concentrations and temperatures. Furthermore, by examining the interaction between the plant extract and the metal surface, the study hopes to better understand corrosion inhibition mechanisms. To look for any obvious changes and evaluate the plant extract’s protective qualities, morphological analysis of the mild steel surface was performed both before and after the inhibitor treatment.
The use of mango peel as a corrosion inhibitor in this study promotes an economical and environmentally friendly approach to addressing corrosion-related issues. The findings of this study may help to develop environmentally friendly corrosion inhibitors, as well as provide valuable information about the use of plant extracts in mild steel corrosion control in corrosive environments. Finally, using mango peel as a corrosion inhibitor can help to recycle agricultural waste materials for valuable industrial applications while also meeting the global demand for sustainable corrosion control solutions.
Materials and methods
Materials and equipment
The materials used in this investigation include rectangular mild steel bars, Mangifera indica peels, 1.0 M hydrochloric acid (HCl), acetone, and absolute ethanol. Whatman filter paper, an electronic blender (Model: SB-1872), a thermostat water bath (Model: KW-1000DC), beakers (Model: JPI 8022), emery paper with grit sizes, an electronic compact scale (Model: BL20001) with an accuracy of 0.001 g, a bulk scientific infrared spectrophotometer (Model: M752N), and a digital veneer caliper (150 mm, 6”) were among the tools used.
Materials sourcing and preparation
Rectangular mild steel bars obtained from InnoChris Chemicals in Bridge Head Onitsha, Nigeria, were used as the metal coupon for this investigation. Fresh Mangifera indica peels were sourced from Eke-Awka Market in Awka, Nigeria, and absolute ethanol, acetone, and 1.0 M hydrochloric acid were obtained from PriceChem Ltd. in Onitsha, Nigeria.
The coupons were cut to a size of 2 cm x 1 cm x 0.5 cm. prior to the experiment, the mild steel coupons were ground and polished using an electric grinder and 600 µm – 1000 µm emery paper. The coupons were thoroughly washed with acetone, dried with an air gun, and stored in moisture-free desiccators. Before using each coupon in the immersion test or weight loss method, its weight was determined with an analytical balance.
Fresh Mangifera indica peels were thoroughly washed in distilled water and sun-dried for approximately two weeks. After drying, the peels were ground into powder with an electric blender and sieved to obtain fine particles. To extract bioactive compounds from peels, a solvent extraction method was used. The fine particles were then soaked in 500 ml of 1.0 M absolute ethanol solution for 48 hours before being filtered through Whatmann filter paper to form the corrosion inhibitor. The filtrate was completely dried in a digital water bath at 70 oC for 6 hours and ground to powder. The dried powder was then ready for use in preparing a corrosion inhibitor solution for a weight loss or immersion test. The prepared 1.0 M HCl solution and the required extract concentrations of 1.0, 1.5 g, and 2.0 g were measured on an electronic compact scale.
Weight loss measurement
For each set of experiments, the prepared mild steel coupons were weighed on an electronic compact scale before being immersed in 40 ml of 1.0 M HCL with and without Mangifera indica peel extract at concentrations of 1.0, 1.5, and 2.0 g/L in 100 ml beakers that were properly labeled. The solution temperatures of 303 K and 323 K were held for 1, 2, 4, 6, and 8 hours, respectively, while the beakers were immersed in a thermostated water bath. After completing the immersion test, the mild steel coupons were washed with distilled water and acetone. The coupons were weighed and dried. The rate of corrosion for mild steel was determined by measuring weight loss. The rate of corrosion and percentage inhibition efficiency were calculated using Equations 1 and 2, respectively.
Corrosion rate (mm/yr) = (1)
Where; k = 8.76 x 104, W = weight loss (g), A = area in of the coupon (cm2), t = immersion time (hr), and D = density of mild steel (g/cm3).
Inhibition efficiency, IE (%) = (2)
Where CRo = corrosion rate of uninhibited metal (g) and CRf = corrosion rate of inhibited metal (g).
Results and discussion
Chemical composition of mild steel
Table 1 displays the chemical composition of the mild steel sample. The sample has approximately 0.16 wt% carbon, 0.71 wt% manganese, 0.03 wt% sulfur, 0.02 wt% phosphorus, 0.02 wt% silicon, 0.01 wt% chromium, and an iron balance.
Corrosion rate
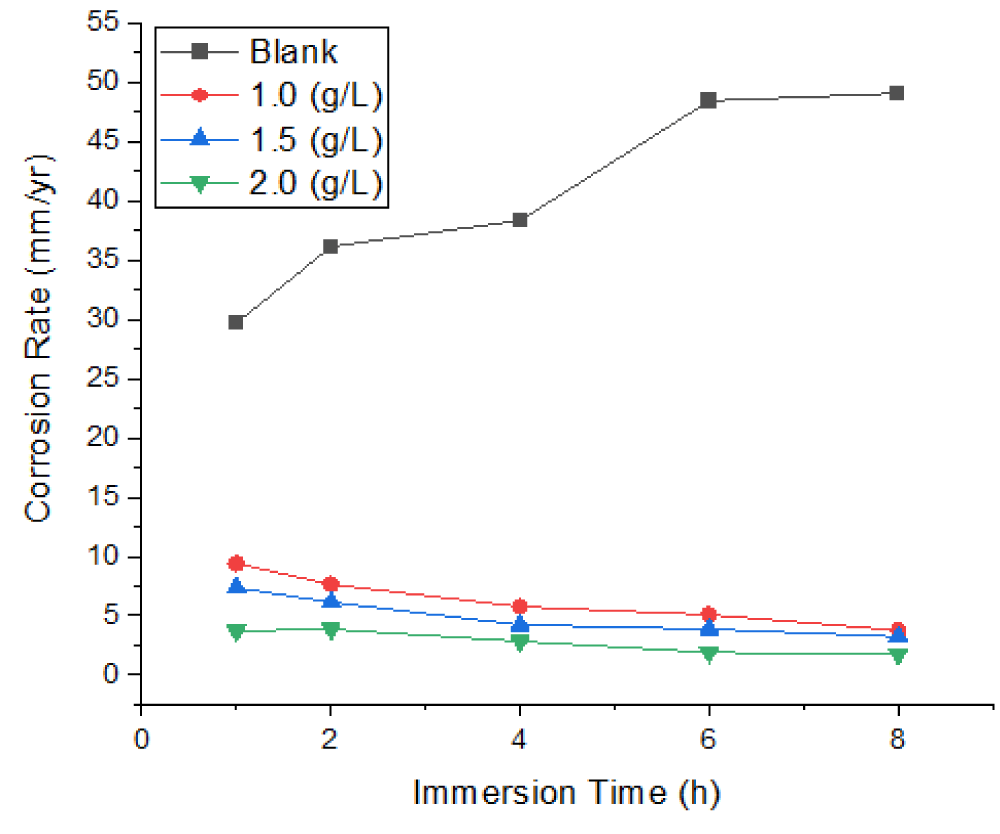
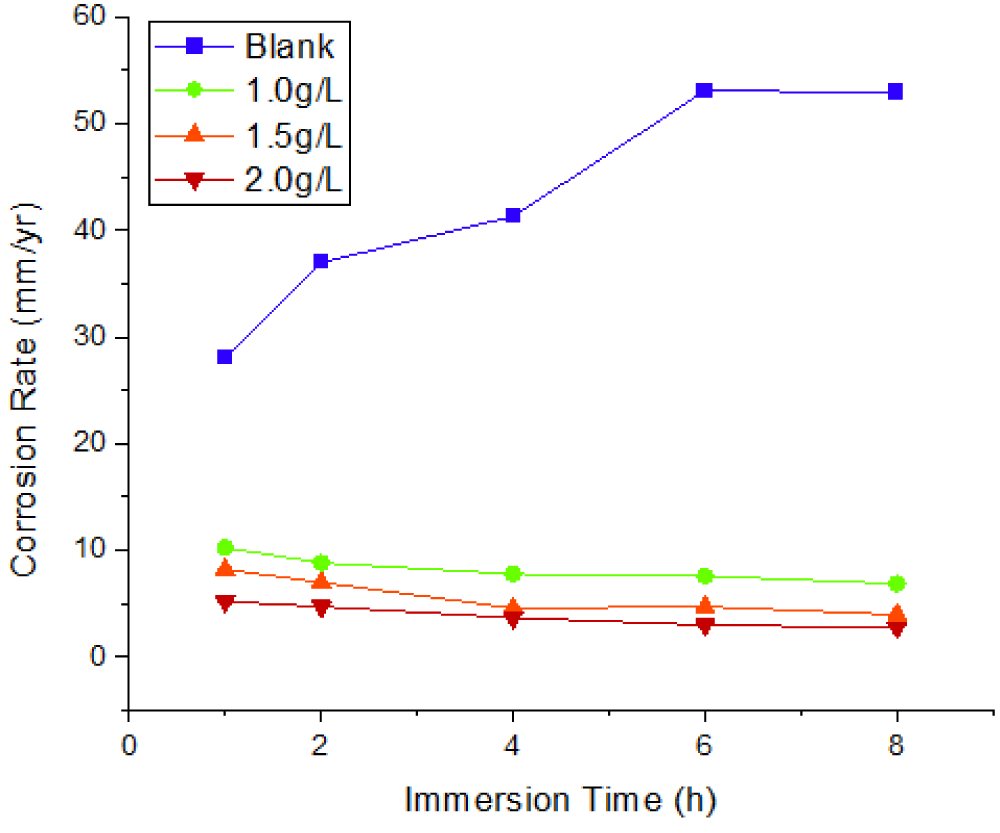
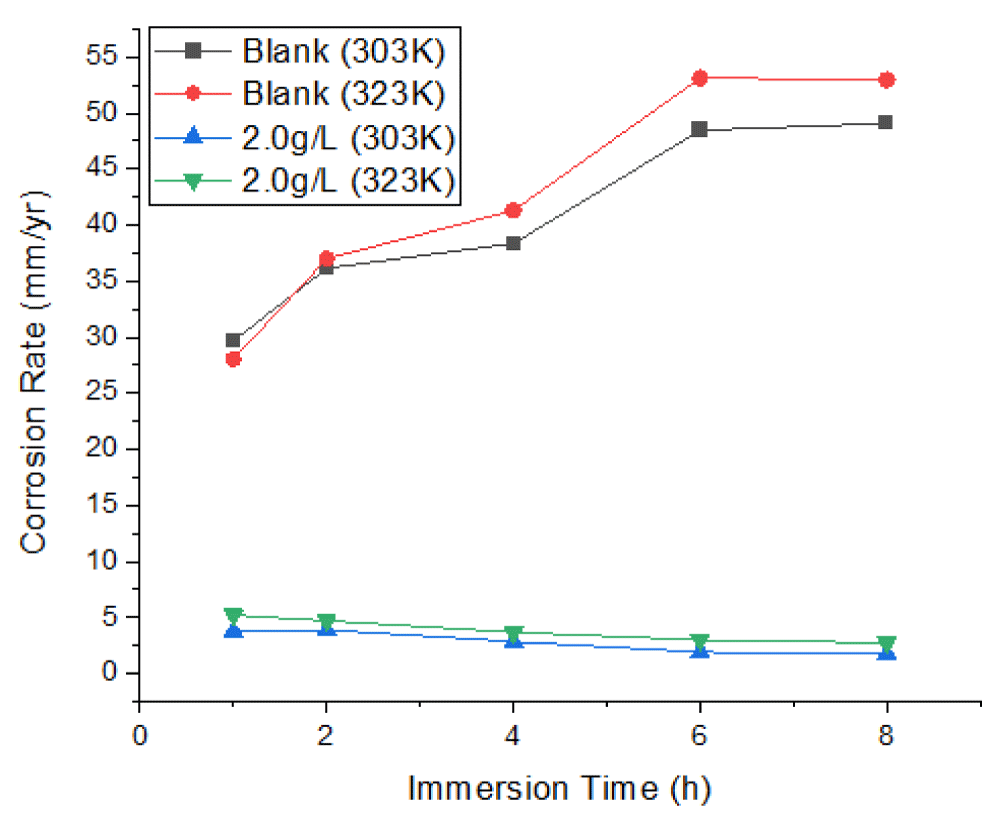
Figures 1-3 show the corrosion parameters of mild steel immersed in a 1 M HCl solution containing 0, 1.0, 1.5, and 2.0 g/L Mangifera Indica Peels Extract (MIPE) at solution temperatures of 303 K and 323 K, respectively, and immersion times (IT) of 1, 2, 4, 6, and 8 h. The temperature and extract concentrations had a significant impact on how mild steel corroded. The rate of corrosion is directly proportional to the increase in solution temperature and inversely proportional to extract concentration. These occurrences support Wei, et al.’s [7] findings. At various solution temperatures, adding a concentration of Mangifera Indica Peel Extract (MIPEC) significantly reduced the corrosion reaction of mild steel. Lower temperatures and higher concentrations of fresh Mangifera indica peel extract increased its inhibitory activity.
Figure 1 depicts the effects of MIPEC and IT on the corrosion rate of mild steel in 1 M HCl at 303 K; Figure 2 depicts the effects of MIPEC and IT on the corrosion rate of mild steel in 1 M HCl at 323 K; and Figure 3 depicts the effects of temperature on the corrosion rate of uninhibited and inhibited mild steel immersed in 1 M HCl at solution. Figures 1–3 show the effects of MIPE on the corrosion behavior of mild steel at various temperatures and immersion times (IT) in 1 M HCl. In the acidic medium, uninhibited mild steel corroded at a rate of up to 29.6809 mm/yr at 303 K/1.0 h IT. The corrosion rate of uninhibited mild steel increased dramatically as temperature and IT increased. At solution temperatures of 303 K and 323 K, and an IT of 8 hours, the maximum corrosion rates were 49.0586 mm/yr and 52.9439 mm/yr.
By adding 1.0 g/L of MIPE to the 1 M HCl solution, the mild steel’s corrosion rate was significantly reduced. At solution temperatures of 303 K and 323 K 1 h IT, corrosion rates decreased from 29.6809 mm/yr and 28.0320 mm/yr to 9.4001 mm/yr and 10.2546 mm/yr. At 303 K and an IT of 1 hour, the corrosion rates of mild steel inhibited by 1.0 g/L MIPE were reduced by 68.3% and 63.4%, respectively. The corrosion rate of mild steel decreased as extract concentration increased at IT and solution temperatures. As extract concentrations and IT levels increased, the rate of corrosion decreased. These phenomena agree with the findings of Zhang, et al. [29], Zhao, et al. [30], and Wei, et al. [7]. At lower temperatures and longer IT at 2.0 g/L MIPE, the inhibited mild steel exhibited the lowest corrosion rate, representing a 96.26% reduction in corrosion rate. The decreased surface roughness and pitting could be attributed to a significant decrease in corrosion rate. The corrosion rate of the inhibited mild steel decreased as immersion time increased, whereas the corrosion rate increased systematically with immersion time when no inhibitor was present. This is evident from an examination of Figures 1-3. The phytochemical components of the extract that have been adsorbed onto the mild steel surface act as a barrier at the interface between the mild steel and the hydrochloric acid solution, slowing the rate of corrosion.
Corrosion inhibition efficiency
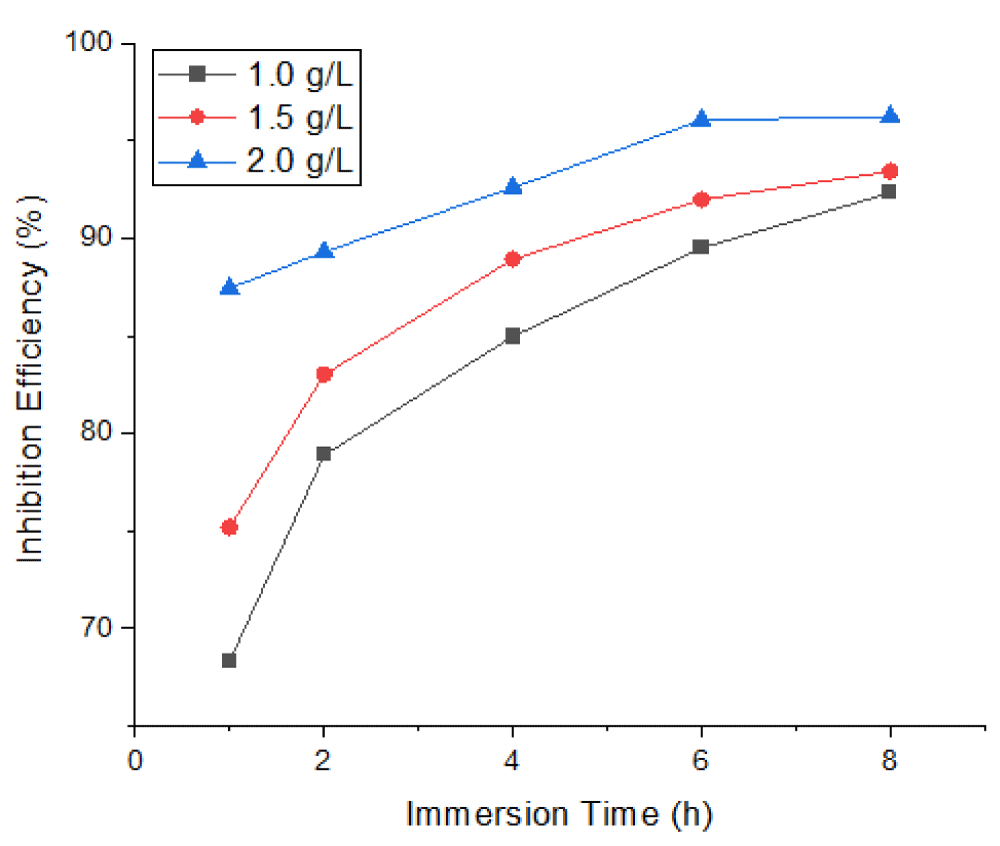
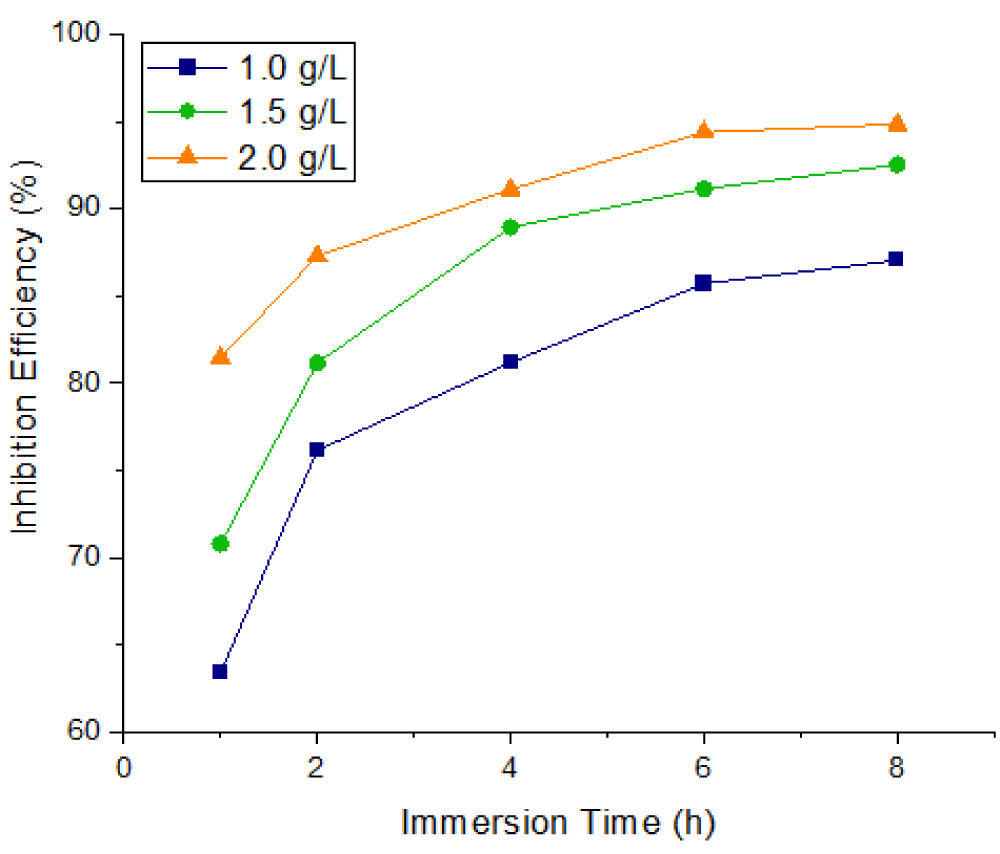
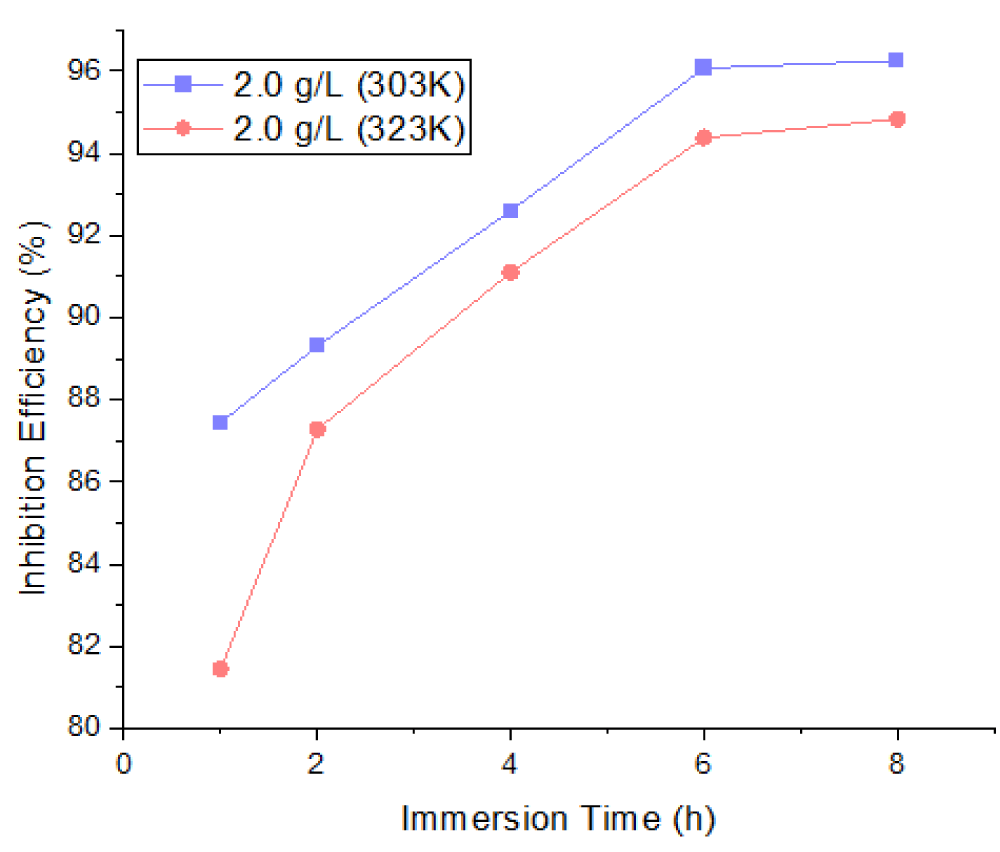
The inhibition characteristics of Mangifera Indica Peel Extract (MIPE) on mild steel at temperatures of 303 K and 323 K in a 1 M HCl solution at different immersion times—1, 2, 4, 6, and 8 hours—are shown in Figures 4-6. In 1 M HCl, the extract from the peels of Mangifera indica exhibited efficient inhibition; the maximum inhibition efficiency was observed at lower temperatures and higher extract concentrations. Higher temperatures cause the extract molecules to dissolve, making it more difficult for them to adsorb on mild steel surfaces.
Figure 4 shows the effects of MIPEC and IT on the inhibition efficiency of mild steel in 1 M HCl at 303 K; Figure 5 shows the effects of MIPEC and IT on the inhibition efficiency of mild steel in 1 M HCl at 323 K; and Figure 6 shows the effects of temperature on the inhibition efficiency of MIPE on mild steel immersed in 1 M HCl solution. The inhibition properties of the peel extract of Mangifera indica on mild steel submerged in 1 M HCl at different times and temperatures are shown in Figures 4-6. The extract exhibited a significant inhibition, as evidenced by its ability to reach up to 68.33% and 63.42%, respectively, at 1.0 g/L extract concentration, immersion times of 1 hour, and solution temperatures of 303 K and 323 K. With increasing extract concentrations and immersion times, the inhibition efficiency gradually rose. The extract’s inhibition potential decreases with increasing temperature because its adsorption coefficient on the mild steel surface decreases, promoting cathodic and anodic reactions. At greater concentrations, lower solution temperatures, and longer immersion times, the MIPE demonstrated a dominant effect. Maximum inhibition potentials of 96.26% and 94.83% were recorded by the 2.0 g/L MIPE at solution temperatures of 303 K and 323 K, with an 8-hour immersion period. Comparatively, MIPE shows better inhibition potential in HCl solution than in seawater. It also showed a better inhibitive effect than Mikania micrantha [31] and rice straw [32] in H2SO4 solution.
The ability of MIPE to inhibit is contingent upon the phytochemical constituents’ adsorption onto the mild steel surface. The active centers on a mild steel surface were blocked by the insoluble complex that adsorbed on it, increasing the surface coverage where a steady inhibition was present. When the temperature increased while the extracts were present, the inhibition efficiency dropped. The active center on the mild steel surface was exposed to the corrosive medium at high temperatures, which accelerated the rate of corrosion by increasing the rate of mild steel dissolution and desorption of the adsorbed inhibitor constituents from the metal surface. At higher temperatures, desorption of the inhibitor film occurs in parallel with adsorption, exposing the mild surface to severe attack.
Conclusion
The study looked at how temperature and the concentration of Mangifera Indica Peel Extract (MIPE) affected mild steel corrosion in a 1 M HCl solution. The study confirmed that MIPE is a highly effective natural corrosion inhibitor, offering a more sustainable and environmentally friendly alternative to conventional inhibitors for protecting mild steel in acidic environments. MIPE significantly reduced the corrosion rate of mild steel in 1 M HCl solution. The corrosion rate of uninhibited mild steel increased with higher solution temperatures and longer immersion times. In contrast, the inhibited mild steel showed a more effective decrease in corrosion rate at lower temperatures. With longer immersion times and higher extract concentrations, the efficiency of inhibition rose, reaching maximum inhibition potentials of 94.83% and 96.26% at 2.0 g/L MIPE and 323 and 303 K, respectively. Further research should focus on optimizing the concentration of MIPE for maximum corrosion inhibition efficiency, as well as investigating the long-term effects of MIPE on corrosion inhibition over extended immersion times to provide critical knowledge about the sustainability and longevity of the extract’s protective layer.
This work was supported by Nnamdi Azikiwe University, through the Tertiary Education Trust Fund (TETfund), Institution Based Research grant (IBR), 2023.
- Karattu Veedu K, Peringattu Kalarikkal T, Jayakumar N, Gopalan NK. Anticorrosive performance of Mangifera indica L. leaf extract-based hybrid coating on steel. ACS Omega. 2019;4(6):10176-10184. Available from: https://doi.org/10.1021/acsomega.9b00632
- Ramezanzadeh M, Bahlakeh G, Sanaei Z, Ramezanzadeh B. Corrosion inhibition of mild steel in 1 M HCl solution by ethanolic extract of eco-friendly Mangifera indica (mango) leaves: electrochemical, molecular dynamics, Monte Carlo and ab initio study. Appl Surf Sci. 2019;463:1058-1077. Available from: https://doi.org/10.1016/j.apsusc.2018.09.029
- Bender R, Féron D, Mills D, Stefan R, Ralph B, Dirk B, et al. Corrosion challenges towards a sustainable society. Mater Corros. 2022;73(11):1730-1751. Available from: https://pure.tudelft.nl/ws/portalfiles/portal/139174690/Materials_Corrosion_2022_Bender_Corrosion_challenges_towards_a_sustainable_society.pdf
- Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B. Study of the synergistic effect of Mangifera indica leaves extract and zinc ions on the mild steel corrosion inhibition in simulated seawater: computational and electrochemical studies. J Mol Liq. 2019;292:111387. Available from: https://doi.org/10.1016/j.molliq.2019.111387
- Yahya S. Corrosion inhibition efficiency of steel by mango peel extract in hydrochloric acid at different temperature. Sci Res J. 2023;75-96. Available from: https://doi.org/10.24191/srj.v20is.23289
- Patel J, Patel DD. Corrosion inhibition by Musa paradisiaca peel extract for SS 304 in 1 M hydrochloric acid solution. Indian J Sci Technol. 2024;17(18):1854-1859. Available from: https://doi.org/10.17485/IJST/v17i18.570
- Wei G, Deng S, Li X. Eupatorium adenophora (Spreng.) leaves extract as a highly efficient eco-friendly inhibitor for steel corrosion in trichloroacetic acid solution. Int J Electrochem Sci. 2022;17(11):221182. Available from: https://doi.org/10.20964/2022.11.63
- Tang M, Deng S, Du G, Li X. Mikania micrantha extract/KI blend as a novel synergistic inhibitor for steel corrosion in concentrated H3PO4 solution. Ind Crops Prod. 2023;193. Available from: https://doi.org/10.1016/j.indcrop.2023.116237
- El Ibrahimi B, Jmiai A, Bazzi L, El Issami S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arabian J Chem. 2020;13(1):740-771. Available from: https://doi.org/10.1016/j.arabjc.2017.07.013
- Ogunleye OO, Eletta OA, Arinkoola AO, Agbede OO. Gravimetric and quantitative surface morphological studies of Mangifera indica peel extract as a corrosion inhibitor for mild steel in 1 M HCl solution. Asia-Pac J Chem Eng. 2018;13(6). Available from: https://doi.org/10.1002/apj.2257
- Emmanuel JK. Corrosion protection of mild steel in corrosive media, a shift from synthetic to natural corrosion inhibitors: a review. Bull Natl Res Cent. 2024;48(1). Available from: https://bnrc.springeropen.com/articles/10.1186/s42269-024-01181-7
- Mbamalu EE, Chinedu AP. Assessment of the corrosion inhibitory potentials of Chromolaena odorata leaf extract on mild steel in hydrogen chloride acid environment. Moroccan J Chem. 2023;11(1):188-204. Available from: http://dx.doi.org/10.48317/IMIST
- Miralrio A, Espinoza Vázquez A. Plant extracts as green corrosion inhibitors for different metal surfaces and corrosive media: a review. Processes. 2020;8(8):942. Available from: https://doi.org/10.3390/pr8080942
- Zhu Y, Wang L, Behnamian Y, Song S, Wang R, Gao Z, ET AL. Metal pitting corrosion characterized by scanning acoustic microscopy and binary image processing. Corros Sci. 2020;170:108685. Available from: http://dx.doi.org/10.1016/j.corsci.2020.108685
- Bhardwaj N, Sharma P, Kumar V. Phytochemicals as steel corrosion inhibitors: an insight into mechanism. Corros Rev. 2021;39(1):27-41. Available from: https://doi.org/10.1515/corrrev-2020-0046
- Odio BO, Chinwuko EC, Chukwuneke JL, Sinebe JE. Investigation of the effect of corrosion on mild steel in five different environments. Int J Sci Technol Res. 2014;3(7):306-310. Available from: https://www.ijstr.org/final-print/july2014/Investigation-Of-The-Effect-Of-Corrosion-On-Mild-Steel-In-Five-Different-Environments.pdf
- Achebe CH, Ilogebe AB, Chukwuneke JL, Ezeanyim OC. The effects of inhibition on corrosion of mild steel in H2SO4 using ethanol extract of Vernonia amygdalina. Int J Eng Sci. 2015;4(8):28-36. Available from: https://www.theijes.com/papers/v4-i8/Version-2/E0482028036.pdf
- Ahmad MF, Mohammed NJ, Othman NK. Electrochemical studies of date palm seed as a green corrosion inhibitor for carbon steel in 0.5 M H2SO4 and CH3COOH conditions. In: Lecture Notes in Mechanical Engineering. Springer; 2023;239-246. Available from: http://dx.doi.org/10.1007/978-981-19-1851-3_21
- Mbah CN, Onah CC, Nnakwo KC. Effectiveness of Irvingia wombolu extract on corrosion inhibition of mild steel in hydrochloric acid solution. Eng Res Express. 2020;2(1):015039. Available from: https://ui.adsabs.harvard.edu/link_gateway/2020ERExp...2a5039M/doi:10.1088/2631-8695/ab78ec
- Chukwuneke J, Olisakwe H, Nnakwo K. Enhancing mechanical properties of aluminium-based biocomposites through the addition of hybrid reinforcing particulates. J Civil Eng Environ Sci. 2024;10(2):050-53. Available from: https://dx.doi.org/10.17352/2455-488X.000083
- Chukwuneke JL, Sinebe JE, Umahi JC, Nnakwo KC, Olisakwe HC. Evaluation of microstructure evolution and mechanical properties of Al-10Zn-1.63Si/Irvingia gabonensis particulates alloy composites. J Appl Biomater Funct Mater. 2024. Available from: https://doi.org/10.1177/22808000241236021
- Espinoza-Vázquez A, Rodríguez-Gómez FJ, Negrón-Silva GE, González-Olvera R, Ángeles-Beltrán D, Palomar-Pardavé M, Miralrio A, Castro M. Fluconazole and fragments as corrosion inhibitors of API 5L X52 steel immersed in 1 M HCl. Corros Sci. 2020;174:108853. Available from: http://dx.doi.org/10.1016/j.corsci.2020.108853
- Ayoola AA, Babalola R, Durodola BM, Alagbe EE, Agboola O, Adegbile EO. Corrosion inhibition of A36 mild steel in 0.5 M acid medium using waste citrus limonum peels. Results Eng. 2022;15:100490. Available from: https://doi.org/10.1016/j.rineng.2022.100490
- Kaur J, Saxena A. Phytochemicals/plant extracts as corrosion inhibitors for steel alloys in H2SO4 solutions. In: Phytochemistry in Corrosion Science. CRC Press; 2024;99-107. Available from: http://dx.doi.org/10.1201/9781003394631-6
- Thakur A, Kumar A, Kaya S, Marzouki R, Zhang F, Guo L. Recent advancements in surface modification, characterization, and functionalization for enhancing the biocompatibility and corrosion resistance of biomedical implants. Coatings. 2022;12(10):1459. Available from: https://doi.org/10.3390/coatings12101459
- Mbah CN, Onyeabor DC, Nebechi CS, Anuta MC, Nnakwo KC. Evaluation of corrosion characteristics of mild steel in acidic environment using cocoyam and almond leaves extracts as inhibitors. J Civil Eng Environ Sci. 2023;9(2):20-24. Available from: http://dx.doi.org/10.17352/2455-488X.000063
- Mbah CN, Nebechi CS, Oko NE, Onyeabor DC, Nnakwo KC, Nweke I. Green corrosion inhibition performance of orange leaves extract on mild steel sheet buried in an acidified soil. Int J Res Adv Eng Technol. 2023;9(2):24-27. Available from: https://www.allengineeringjournal.in/archives/2023/vol9/issue2/9012
- Kaya F, Solmaz R, Geçibesler İH. The use of methanol extract of Rheum ribes (Işgın) flower as a natural and promising corrosion inhibitor for mild steel protection in 1 M HCl solution. J Ind Eng Chem. 2023. Available from: https://doi.org/10.1016/j.jiec.2023.02.013
- Zhang F, Deng S, Wei G, Li X. Alternanthera philoxeroides extract as a corrosion inhibitor for steel in Cl3CCOOH solution. Int J Electrochem Sci. 2023;18(3):100057. Available from: https://doi.org/10.1016/j.ijoes.2023.100057
- Zhao Q, Guo J, Cui G, Han T, Wu Y. Chitosan derivatives as green corrosion inhibitors for P110 steel in a carbon dioxide environment. Colloids Surf B Biointerfaces. 2020;194:111150. Available from: https://doi.org/10.1016/j.colsurfb.2020.111150
- Tang M, Li X, Deng S, Lei R. Synergistic inhibition effect of Mikania micrantha extract with KI on steel corrosion in H2SO4 solution. J Mol Liq. 2021;344. Available from: https://doi.org/10.1016/j.molliq.2021.117926
- Oyewole O, Abayomi TS, Oreofe TA, Oshin TA. Anti-corrosion using rice straw extract for mild steel in 1.5 M H2SO4 solution. Results Eng. 2022;16:100684. Available from: http://dx.doi.org/10.1016/j.rineng.2022.100684
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
